案例详细介绍
Xplus medical has been specializing in research, development and production of medical devices and consumables, which are utilized in the domains of respiratory, emergency and critical care since 2009. Furthermore, most of Xplus medical products have been certified by ISO 9001 & 13485, CE0123 and FDA. Therefore, the quality of all our products has been guaranteed.
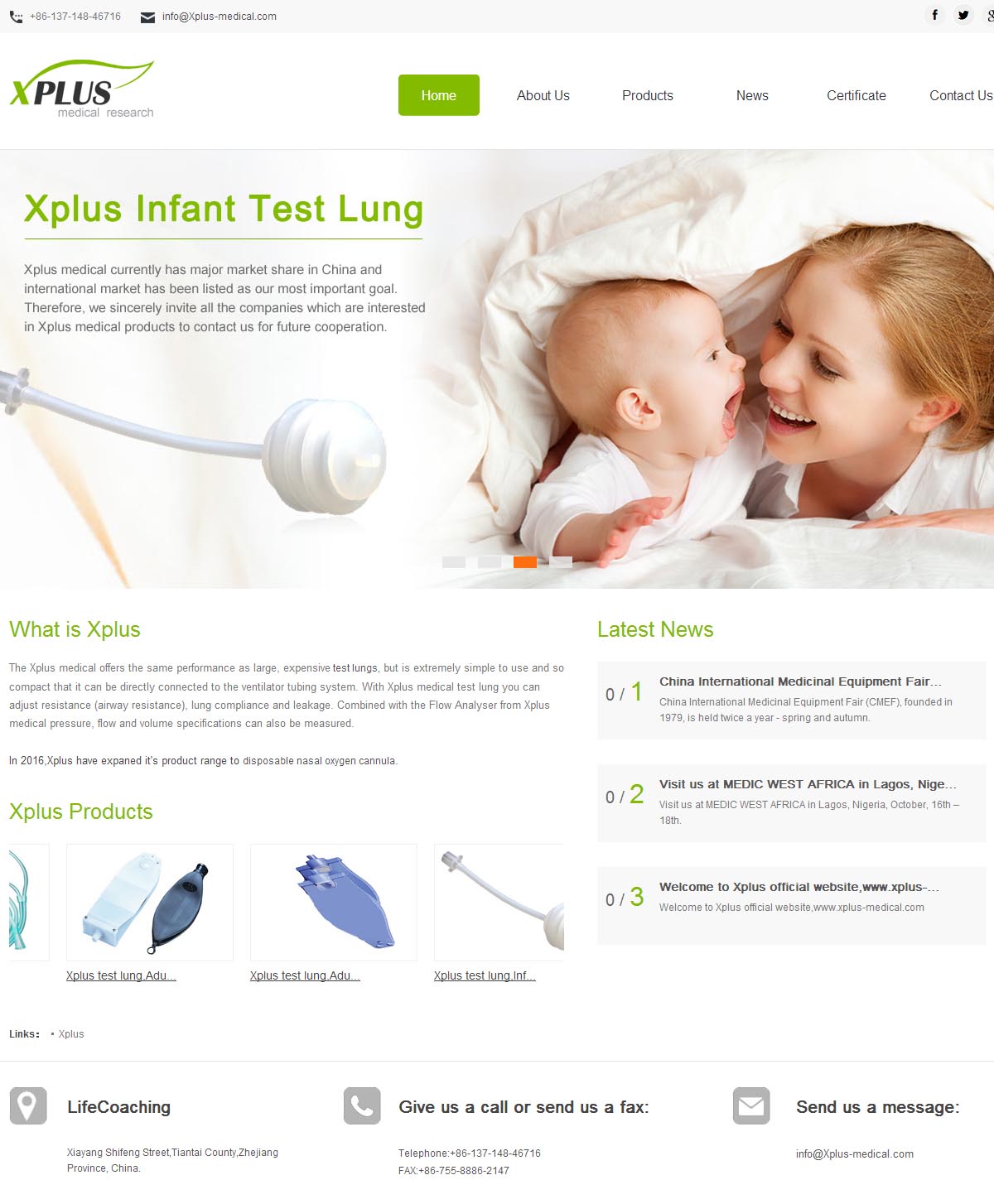
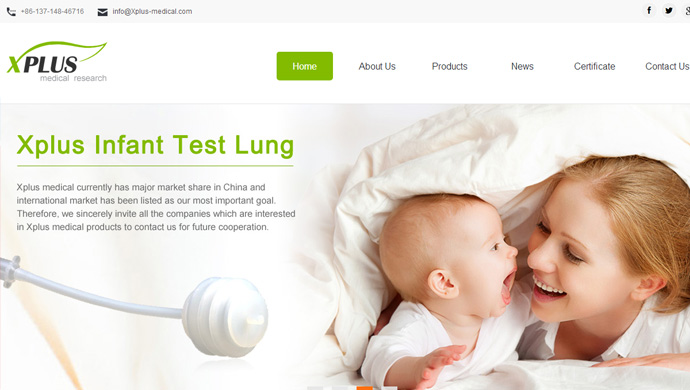
Xplus medical currently has major market share in China and international market has been listed as our most important goal. Therefore, we sincerely invite all the companies which are interested in Xplus medical products to contact us for future cooperation.




 深圳市龙华新区龙华街道办山咀头综合办公大楼9楼915室 (龙华街道办、华润万家,佳华商场、友谊书城等旁)
深圳市龙华新区龙华街道办山咀头综合办公大楼9楼915室 (龙华街道办、华润万家,佳华商场、友谊书城等旁) 